PDF version
Research article
S.H. Khan,1 A. Ijaz,2 S.A. Raza Bokhari,1 M.S. Hanif 1 and N. Azam 1
تواتر خلل تحمل الغلوكوز والسكّري لدى من يقل غلوكوز الدم على الريق لديهم عن 6.1 ميلي مول/لتر (110 ميلي غرام/ديسي لتر)
إسكندر حياة خان، عامر إعجاز، سيد عون رضا شاه يبخار، محمد شِهزاد حنيف، نائلة أعظم
الخلاصـة: لا يوجد اتفاق على تشخيص السكّري وفق المعايير المتاحة، ويعتمد إلى حدّ كبير على نتائج غلوكوز الدم الصيامي (على الريق). وقد أجرَى الباحثون هذه الدراسة في عامَيْ 2010 - 2011، وتهدف لقياس تواتر خلل تحمل الغلوكوز والسكّري لدى 127 شخصاً يقل لديهم غلوكوز الدم الصيامي عن 7 ميلي مول/لتر، وكذلك لقياس التوافق بين معايير التشخيص القياسية المختلفة. ووضع الباحثون المراجعين للمختبر من أجل تحليل غلوكوز الدم الصيامي أمام تحدٍ بالغلوكوز الفموي مقداره 75 غراماً على مدى ساعتين، من أجل استبعاد تشخيص السكري؛ فوجدوا أن مجمل %40.6 من المراجعين الذين كان مستوى غلوكوز الدم الصيامي لديهم يصل إلى 5.6 - 6.0 ميلي مول/لتر كان لديهم تنظيم غير سوي للغلوكوز وفقاً للمعيار الذهبي للتحدي بالغلوكوز. وكان التوافق بين معايير الجمعية الأمريكية للسكّري ومنظمة الصحة العالمية مقبولاً (كابا = 0.32). ويرى الباحثون أن حالات اضطراب استقلاب الغلوكوز بما فيها خلل تحمل الغلوكوز، والسكّري يمكن أن تُصادَف ضمن مجموعة نتائج غلوكوز الدم الصيامي التي تقل عن 6.1 ميلي مول/لتر (110 ميلي غرام/ديسي لتر).
ABSTRACT The diagnosis of diabetes mellitus by the available criteria is controversial and relies heavily on fasting glucose results. This cross-sectional study in 2010–2011 aimed to measure the frequency of impaired glucose tolerance and diabetes mellitus in 127 subjects having fasting blood glucose < 7.0 mmol/L and to measure the agreement between different standard diagnostic criteria. Subjects presenting to a laboratory for analysis of fasting blood glucose for excluding diabetes mellitus underwent a 2-hour 75 g oral glucose challenge. A total of 40.6% of subjects with fasting blood glucose from 5.6–6.0 mmol/L had abnormal glucose regulation on the basis of the gold standard glucose challenge. Agreement between American Diabetes Association and World Health Organization diagnostic criteria was only fair (kappa = 0.32). Abnormalities of glucose metabolism including impaired glucose tolerance and diabetes mellitus can exist at fasting blood glucose results < 6.1 mmol/L (110 mg/dL).
Fréquence de la diminution de la tolérance au glucose et du diabète chez des sujets ayant une glycémie à jeun inférieure à 6,1 mmol/l (110 mg/dl)
RÉSUMÉ Le diagnostic du diabète selon les critères disponibles est controversé et repose principalement sur les résultats de la glycémie à jeun. La présente étude transversale menée en 2010 et 2011 visait à mesurer la fréquence de la diminution de la tolérance au glucose et du diabète chez 127 sujets présentant une glycémie à jeun inférieure à 7,0 mmol/l et à mesurer la concordance entre différents critères diagnostiques standard. Les sujets se présentant au laboratoire pour une analyse de la glycémie à jeun visant à éliminer un diagnostic de diabète ont passé une épreuve d'hyperglycémie provoquée par voie orale deux heures après l'ingestion de 75 g de glucose. Au total, 40,6 % des sujets ayant une glycémie à jeun entre 5,6–6,0 mmol/l présentaient une régulation anormale de la glycémie selon les critères de référence de l'épreuve d'hyperglycémie. La concordance entre les critères diagnostiques de l'American Diabetes Association et de l'Organisation mondiale de la Santé était seulement passable (kappa = 0,32). Les anomalies du métabolisme du glucose telles que la diminution de la tolérance au glucose et le diabète sont possibles avec des résultats de la glycémie à jeun inférieurs à 6,1 mmol/l (110 mg/dl).
1Department of Pathology, PNS Rahat Hospital, Karachi, Pakistan (Correspondence to S.H. Khan: This email address is being protected from spambots. You need JavaScript enabled to view it.).
2Department of Pathology, PNS Shifa Hospital, Karachi, Pakistan.
Received: 01/11/11; accepted: 05/02/12
EMHJ, 2013, 19(2):175-180
Introduction
Diabetes mellitus (DM) is one of the leading global causes of mortality and is a growing epidemic [1]. While the causes of the disease, i.e. sedentary lifestyles, higher intake of refined carbohydrates and saturated fatty acids, synergize to add to the disease burden, the situation is aggravated by the unavailability of any curative treatments [2]. Added to this, delays in diagnosis make subjects vulnerable to various complications of the disease [3].
The diagnosis of DM relies on the demonstration of raised glucose levels in the patient’s blood. However, this is more complex than it seems due to existence of multiple diagnostic criteria. Traditionally an oral glucose tolerance test (OGTT) was used to diagnose DM [4]. The latest World Health Organization (WHO) criteria, however, recommended a 2-step strategy, i.e. an initial fasting glucose test and, if levels are between 6.1–6.9 mmol/L, followed by a 2-hour OGTT [5]. The 2003 American Diabetic Association (ADA) guidelines proposed a fasting glucose result with an upper cut-off of 5.6 mmol/L [6]. Moreover, the published data dealing with the labelling of hyperglycaemia suffer from the problems of different diagnostic cut-offs and prevalence rates for DM [7,8].
Apart from the lack of consensus regarding the diagnosis of DM, other controversies exist. First, clinical practice suggests that subjects having fasting glucose levels < 6.1 mmol/L or even < 5.6 mmol/L may receive a diagnosis of DM in an OGTT and vice versa [9]. Secondly, the evidence also suggests a definite lag in the diagnosis of DM, resulting in subjects being at risk of various DM-related complications from the outset [10]. Thirdly, the literature has highlighted the risks associated with a decision based on a single reading of fasting blood glucose on account of patient non-compliance with fasting or laboratory-related inaccuracies [11]. Finally, older subjects may be normoglycaemic, but can become glucose intolerant on exposure to a glucose load [12].
This study in Karachi, Pakistan was therefore designed to measure the frequency of impaired glucose tolerance (IGT) and DM in subjects having fasting blood glucose < 7.0 mmol/L and to measure the agreement between the ADA and the WHO diagnostic criteria for DM.
Methods
Study setting and sample
This cross-sectional study was carried out at the department of pathology at PNS Rahat and Shifa hospitals, Karachi, from March 2010 to June 2011.
All subjects who presented to the laboratory for analysis of fasting blood glucose for excluding DM were eligible for the study. Subjects were initially interviewed for clinical details (e.g. history of hypertension, DM and ischaemic heart disease). Subjects who gave a history of intake of medicines, were known diabetics, were not observing proper medical fasting, were inpatients, were pregnant or had some physical or mental stress were excluded from the study.
Data collection
After explanation of the study prerequisites and obtaining a signed written consent, 172 subjects were sampled for fasting blood glucose. After the blood samples were taken subjects were requested to undergo a complete a 2-hour 75-g glucose challenge. They were asked to drink the glucose solution (300 mL) over 5 minutes. Starting from the time of intake of glucose, subjects were advised to return for the 2-hour sample for glucose. All samples were analysed within 3 (± 1) hours. Glucose was analysed by the hexokinase method on a random access clinical chemistry analyser (Hitachi-902).
Subjects were grouped into 5 groups according to their fasting blood glucose results: < 5.0 mmol/L ( 6.9 mmol/L (125 mg/dL).
Subjects were defined from 2-hour OGTT results as: normal glucose tolerance 199 mg/dL).
Statistical analysis
All data were entered into SPSS, version 15. Measures of central tendency and dispersion i.e., mean and standard deviation (SD) was calculated for age. Frequencies by sex were also calculated. Frequency of normal glucose tolerance, IGT and DM based upon the 2-hour OGTT readings were measured at various levels of fasting blood glucose defined cut-offs by utilizing descriptive statistics. The differences in 2-hour post-75-g glucose challenge based diagnosis, i.e. normal, IGT and DM across various levels (defined groups), were compared by 1-way ANOVA. Level of agreement between WHO and ADA criteria was measured by the kappa statistic.
Results
The mean age of our study population was 43.7 (SD 10.8) years. Out of the evaluated subjects, 139 were male and 33 were females.
Participants with IGT and DM based on the 2-hour OGTT results spanned all the ranges of fasting blood glucose results (Figure 1 ). An increasing frequency of diagnosis of IGT and DM was seen after the fasting blood glucose results crossed the level of 5.6 mmol/L.
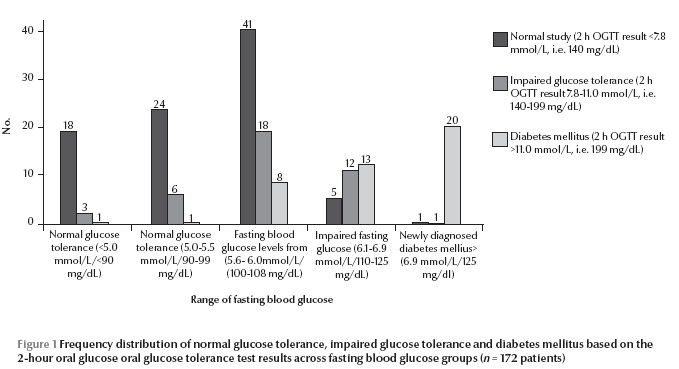
Table 1 shows that the mean 2-hour OGTT results crossed the WHO and ADA defined cut-off of 7.8 mmol/L in subjects having fasting blood glucose results > 5.6 mmol/L.
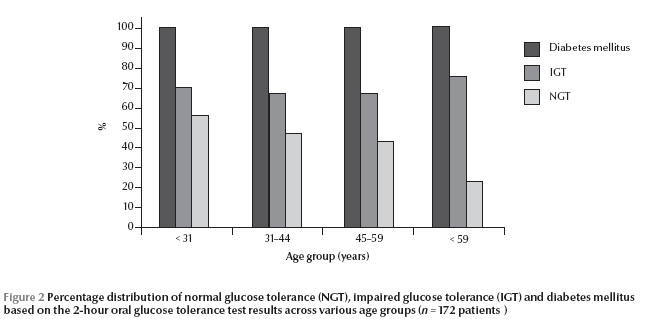
The relative percentage of diagnosis rates of IGT and DM based upon 2-hour OGTT increased with age (Figure 2).
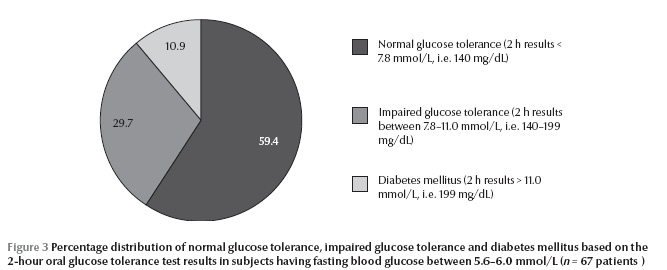
A total of 40.6% of subjects (29.7% IGT and 10.9% DM) with fasting blood glucose between 5.6–6.0 mmol/L had abnormal glucose regulation on the basis of the gold standard 2-hour OGTT results (Figure 3). The agreement between the ADA and the WHO diagnostic criteria was only fair (kappa = 0.32, P < 0.001).
Discussion
Our study has shown that subjects having fasting blood glucose < 6.1 mmol/L can have biochemically abnormal response in terms of glucose intolerance after subjecting them to a glucose load. A review of the literature revealed other studies that agree with our findings [11,13]. We also showed that even subjects in the normal range of fasting glycaemia, i.e. fasting blood glucose < 5.6 mmol/L, can have glucose dysregulation demonstrated during a 2-hour glucose challenge test. Therefore the current diagnostic approach may miss as many as 40% of cases with abnormal glucose metabolism as depicted by a 2-hour OGTT reading, i.e. IGT and DM. Similar results have been shown by studies in which subjects with normal glucose levels and abnormal 2-hour glucose load readings [14]. Richard et al. showed no reliable cut-off for fasting glucose was sensitive enough to rule out DM, and concluded that OGTT testing must be maintained for the diagnosis of DM [8].
The possible reasons for such findings could be multifold. First, fasting and post-glucose challenge results demonstrate 2 different processes; the former may reflect the glycaemic baseline being influenced by the amount of fasting and associated physical and psychological state, while the later reflects a stimulated response from the beta cells of pancreas after glucose loading [15]. Traditionally, stimulated or dynamic glucose testing has been referred as the gold standard. So both glucose results represent different dimensions to the diabetogenic processes [15–17]. Secondly, the trade-off between sensitivity and specificity of the test is the underlying consideration once decisions are required for screening or devising a confirmatory methodology [18]. Our results suggest that sole reliance on fasting blood glucose will compromise sensitivity due to the high number of false negative cases. Once a 2-hour OGTT reading is used, it not only adds strength to the diagnosis but also adds to the sensitivity by identifying subjects with glucose intolerance who have underlying atherosclerosis-related complications [19]. Thirdly, 2-hour readings have been shown to be better correlated with markers of glycation including glycosylated haemoglobin and fructosamine [20]. So why not incorporate a single 2-hour reading to screen subjects suspected of having DM? The quality and cost added by early diagnosis of DM can be weighed against the cost of late initiation of treatment. Already the literature recommends the use of OGTT in the detection of undiagnosed DM in certain disease categories such as stroke and myocardial infarction [21,22]. Philips et al. have recommended a glucose challenge test to improve the diagnostic sensitivity for undiagnosed DM [23]. The question arises as to why wait for the development of complications whose treatment cost greatly exceeds the screening cost? Finally, a review of certain regional literature on diabetes screening highlights the varying recommendations about screening in our population in contrast to their non-Asian counterparts [12,24]. More regional literature do exist recommending a lower threshold of screening for DM in our population [24–26]. Thus racial and regional differences in the pattern of diabetes could be another reason for subjects demonstrating glucose intolerance during glucose loading.
Comparing WHO criteria for diagnosis of diabetes with ADA criteria shows minimal agreement between the 2 definitions of similar disease. The available literature review does suggest comparable results [7,8,27]. In the opinion of the authors, the lower cut-offs may be more compatible with the screening concept for diagnosing DM, while subjects having a slightly higher degree of clinical suspicion may be further confirmed by adopting the 2-hour OGTT test as per WHO recommendations. There may also be an argument for further lowering the cut-offs for our population, but prospective clinical trials must address this issue [18].
We have also observed that ageing is related to post-glucose load intolerance. Choi et al. reported similar findings [12]. The reason could be the age-related deterioration in the functioning of beta cells of the pancreas or the development of insulin resistance which appears later in life [12,28].
There are some limitations to our study which should be noted. First, stress hyperglycaemia is a known entity. Attempts were made to exclude subjects with any degree of physical ailments, but a psychological stress evaluation was not carried out among our subjects. Secondly, the results may have been confounded by the presence of obesity, hypertension or ischaemic heart disease.
This study was clinically important because it highlighted controversies associated with the diagnostic criteria for DM. At present clinical practice suggests labelling of individuals as normoglycaemic based on a fasting blood glucose result. However, in the light of our findings it can be suggested that a patient can have DM when the fasting blood glucose result is normal. Interpretation of fasting blood glucose results must include consideration of a patient’s age and clinical information. Moreover, a 2-hour OGTT reading should also be considered as a screening test after a necessary cost–benefit analysis and consensus among the authorities.
Conclusion
Abnormalities of glucose metabolism including impaired glucose tolerance and DM can exist at fasting blood glucose results < 6.1 mmol/L (110 mg/dL). WHO and ADA criteria showed poor agreement between each other for the diagnosis of DM.
References
- Farag YM, Gaballa MR. Diabesity: an overview of a rising epidemic. Nephrology, Dialysis, Transplantation, 2011, 26:28–35.
- Chang K. Comorbidities, quality of life and patients' willingness to pay for a cure for type 2 diabetes in Taiwan. Public Health, 2010, 124:284–294.
- Baldé NM et al. Frequency of diabetic microangiopathy in newly diagnosed diabetes mellitus in Conakry: late diagnosis and lack of screening. Dakar Med., 2007, 52:165–170.
- National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes, 1979, 28:1039–1057.
- Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia. Report of a WHO/IDF consultation. Geneva, World Health Organization, 2006.
- Summary of revisions for the 2010 clinical practice recommendations. Diabetes Care, 2010, 33(Suppl. 1):S3.
- Herdzik E et al. Comparison of ADA and WHO diagnostic criteria for diabetes diagnosis and other categories of glucose intolerance. Polski Merkuriusz Lekarski, 2002, 13:316–320.
- Richard JL et al. Diagnosis of diabetes mellitus and intermediate glucose abnormalities in obese subjects based on ADA (1997) and WHO (1985) criteria. Diabetic Medicine, 2002, 19:292–299.
- Tirosh A et al. Normal fasting plasma glucose levels and type 2 diabetes in young men. New England Journal of Medicine, 2005, 353:1454–1462.
- Haffner SM et al. Cardiovascular risk factors in confirmed prediabetic individuals. Does the clock for coronary heart disease start ticking before the onset of clinical diabetes? Journal of the American Medical Association, 1990, 263:2893–2898.
- Balion CM et al. Reproducibility of impaired glucose tolerance (IGT) and impaired fasting glucose (IFG) classification: a systematic review. Clinical Chemistry and Laboratory Medicine, 2007, 45:1180–1185.
- Choi KM et al. Comparison of ADA and WHO criteria for the diagnosis of diabetes in elderly Koreans. Diabetic Medicine, 2002, 19:853–857.
- Kuzuya T et al. Committee of the Japan Diabetes Society on the diagnostic criteria of diabetes mellitus. Report of the Committee on the classification and diagnostic criteria of diabetes mellitus. Diabetes Research and Clinical Practice, 2002, 55:65–85.
- De Vegt F et al. Relation of impaired fasting and post load glucose with incident type 2 diabetes in a Dutch population: The Hoorn Study. Journal of the American Medical Association, 2001, 285:2109–2113.
- Li HY et al. The performance of risk scores and hemoglobin A1c to find undiagnosed diabetes with isolated post load hyperglycemia. Endocrine Journal, 2011, 58:441–448.
- Temelkova-Kurktschiev TS, Hanefeld M. Oral glucose tolerance test: to be or not to be performed? Clinical Laboratory, 2002, 48:143–152.
- Silverman RA et al. Hemoglobin A1c as a screen for previously undiagnosed prediabetes and diabetes in an acute-care setting. Diabetes Care, 2011, 34(9):1908–1912.
- Waugh N et al. Screening for type 2 diabetes: literature review and economic modelling. Health Technology Assessment, 2007, 11(17):iii–iv, ix–xi, 1–125.
- Hanefeld M et al. Post-challenge hyperglycaemia relates more strongly than fasting hyperglycaemia with carotid intima-media thickness: the RIAD Study. Risk Factors in Impaired Glucose Tolerance for Atherosclerosis and Diabetes. Diabetic Medicine, 2000, 17:835–840.
- Rosediani M, Azidah AK, Mafauzy M. Correlation between fasting plasma glucose, post prandial glucose and glycated haemoglobin and fructosamine. Medical Journal of Malaysia, 2006, 61:67–71.
- Lindsberg PJ, Tuomi T, Kaste M. Oral glucose tolerance test should be performed after stroke and transient ischemic attack. International Journal of Stroke, 2011, 6:317–320.
- Kitada S et al. Post-load hyperglycemia as an important predictor of long-term adverse cardiac events after acute myocardial infarction: a scientific study. Cardiovascular Diabetology, 2010, 9:75.
- Phillips LS et al. Glucose challenge test screening for prediabetes and undiagnosed diabetes. Diabetologia, 2009, 52:1798–1807.
- Noda M et al. Fasting plasma glucose and 5-year incidence of diabetes in the JPHC diabetes study—suggestion for the threshold for impaired fasting glucose among Japanese. Endocrine Journal, 2010, 57:629–637.
- Ryu S et al. Should the lower limit of impaired fasting glucose be reduced from 110 mg/dL in Korea? Metabolism: Clinical and Experimental, 2006, 55:489–493.
- Nang EE et al. Is there a clear threshold for fasting plasma glucose that differentiates between those with and without neuropathy and chronic kidney disease? The Singapore Prospective Study Program. American Journal of Epidemiology, 2009, 169:1454–1462.
- Park KS et al. Comparison of glucose tolerance categories in the Korean population according to World Health Organization and American Diabetes Association diagnostic criteria. Korean Journal of Internal Medicine, 2000, 15:37–41.
- Hasegawa G. Decreased senescence marker protein-30 could be a factor that contributes to the worsening of glucose tolerance in normal aging. Islets, 2010, 2:258–260.
PDF version
Research article
S.H. Khan,1 A. Ijaz,2 S.A. Raza Bokhari,1 M.S. Hanif 1 and N. Azam 1
تواتر خلل تحمل الغلوكوز والسكّري لدى من يقل غلوكوز الدم على الريق لديهم عن 6.1 ميلي مول/لتر (110 ميلي غرام/ديسي لتر)
إسكندر حياة خان، عامر إعجاز، سيد عون رضا شاه يبخار، محمد شِهزاد حنيف، نائلة أعظم
الخلاصـة: لا يوجد اتفاق على تشخيص السكّري وفق المعايير المتاحة، ويعتمد إلى حدّ كبير على نتائج غلوكوز الدم الصيامي (على الريق). وقد أجرَى الباحثون هذه الدراسة في عامَيْ 2010 - 2011، وتهدف لقياس تواتر خلل تحمل الغلوكوز والسكّري لدى 127 شخصاً يقل لديهم غلوكوز الدم الصيامي عن 7 ميلي مول/لتر، وكذلك لقياس التوافق بين معايير التشخيص القياسية المختلفة. ووضع الباحثون المراجعين للمختبر من أجل تحليل غلوكوز الدم الصيامي أمام تحدٍ بالغلوكوز الفموي مقداره 75 غراماً على مدى ساعتين، من أجل استبعاد تشخيص السكري؛ فوجدوا أن مجمل %40.6 من المراجعين الذين كان مستوى غلوكوز الدم الصيامي لديهم يصل إلى 5.6 - 6.0 ميلي مول/لتر كان لديهم تنظيم غير سوي للغلوكوز وفقاً للمعيار الذهبي للتحدي بالغلوكوز. وكان التوافق بين معايير الجمعية الأمريكية للسكّري ومنظمة الصحة العالمية مقبولاً (كابا = 0.32). ويرى الباحثون أن حالات اضطراب استقلاب الغلوكوز بما فيها خلل تحمل الغلوكوز، والسكّري يمكن أن تُصادَف ضمن مجموعة نتائج غلوكوز الدم الصيامي التي تقل عن 6.1 ميلي مول/لتر (110 ميلي غرام/ديسي لتر).
ABSTRACT The diagnosis of diabetes mellitus by the available criteria is controversial and relies heavily on fasting glucose results. This cross-sectional study in 2010–2011 aimed to measure the frequency of impaired glucose tolerance and diabetes mellitus in 127 subjects having fasting blood glucose < 7.0 mmol/L and to measure the agreement between different standard diagnostic criteria. Subjects presenting to a laboratory for analysis of fasting blood glucose for excluding diabetes mellitus underwent a 2-hour 75 g oral glucose challenge. A total of 40.6% of subjects with fasting blood glucose from 5.6–6.0 mmol/L had abnormal glucose regulation on the basis of the gold standard glucose challenge. Agreement between American Diabetes Association and World Health Organization diagnostic criteria was only fair (kappa = 0.32). Abnormalities of glucose metabolism including impaired glucose tolerance and diabetes mellitus can exist at fasting blood glucose results < 6.1 mmol/L (110 mg/dL).
Fréquence de la diminution de la tolérance au glucose et du diabète chez des sujets ayant une glycémie à jeun inférieure à 6,1 mmol/l (110 mg/dl)
RÉSUMÉ Le diagnostic du diabète selon les critères disponibles est controversé et repose principalement sur les résultats de la glycémie à jeun. La présente étude transversale menée en 2010 et 2011 visait à mesurer la fréquence de la diminution de la tolérance au glucose et du diabète chez 127 sujets présentant une glycémie à jeun inférieure à 7,0 mmol/l et à mesurer la concordance entre différents critères diagnostiques standard. Les sujets se présentant au laboratoire pour une analyse de la glycémie à jeun visant à éliminer un diagnostic de diabète ont passé une épreuve d'hyperglycémie provoquée par voie orale deux heures après l'ingestion de 75 g de glucose. Au total, 40,6 % des sujets ayant une glycémie à jeun entre 5,6–6,0 mmol/l présentaient une régulation anormale de la glycémie selon les critères de référence de l'épreuve d'hyperglycémie. La concordance entre les critères diagnostiques de l'American Diabetes Association et de l'Organisation mondiale de la Santé était seulement passable (kappa = 0,32). Les anomalies du métabolisme du glucose telles que la diminution de la tolérance au glucose et le diabète sont possibles avec des résultats de la glycémie à jeun inférieurs à 6,1 mmol/l (110 mg/dl).
1Department of Pathology, PNS Rahat Hospital, Karachi, Pakistan (Correspondence to S.H. Khan: This email address is being protected from spambots. You need JavaScript enabled to view it.).
2Department of Pathology, PNS Shifa Hospital, Karachi, Pakistan.
Received: 01/11/11; accepted: 05/02/12
EMHJ, 2013, 19(2):175-180
Introduction
Diabetes mellitus (DM) is one of the leading global causes of mortality and is a growing epidemic [1]. While the causes of the disease, i.e. sedentary lifestyles, higher intake of refined carbohydrates and saturated fatty acids, synergize to add to the disease burden, the situation is aggravated by the unavailability of any curative treatments [2]. Added to this, delays in diagnosis make subjects vulnerable to various complications of the disease [3].
The diagnosis of DM relies on the demonstration of raised glucose levels in the patient’s blood. However, this is more complex than it seems due to existence of multiple diagnostic criteria. Traditionally an oral glucose tolerance test (OGTT) was used to diagnose DM [4]. The latest World Health Organization (WHO) criteria, however, recommended a 2-step strategy, i.e. an initial fasting glucose test and, if levels are between 6.1–6.9 mmol/L, followed by a 2-hour OGTT [5]. The 2003 American Diabetic Association (ADA) guidelines proposed a fasting glucose result with an upper cut-off of 5.6 mmol/L [6]. Moreover, the published data dealing with the labelling of hyperglycaemia suffer from the problems of different diagnostic cut-offs and prevalence rates for DM [7,8].
Apart from the lack of consensus regarding the diagnosis of DM, other controversies exist. First, clinical practice suggests that subjects having fasting glucose levels < 6.1 mmol/L or even < 5.6 mmol/L may receive a diagnosis of DM in an OGTT and vice versa [9]. Secondly, the evidence also suggests a definite lag in the diagnosis of DM, resulting in subjects being at risk of various DM-related complications from the outset [10]. Thirdly, the literature has highlighted the risks associated with a decision based on a single reading of fasting blood glucose on account of patient non-compliance with fasting or laboratory-related inaccuracies [11]. Finally, older subjects may be normoglycaemic, but can become glucose intolerant on exposure to a glucose load [12].
This study in Karachi, Pakistan was therefore designed to measure the frequency of impaired glucose tolerance (IGT) and DM in subjects having fasting blood glucose < 7.0 mmol/L and to measure the agreement between the ADA and the WHO diagnostic criteria for DM.
Methods
Study setting and sample
This cross-sectional study was carried out at the department of pathology at PNS Rahat and Shifa hospitals, Karachi, from March 2010 to June 2011.
All subjects who presented to the laboratory for analysis of fasting blood glucose for excluding DM were eligible for the study. Subjects were initially interviewed for clinical details (e.g. history of hypertension, DM and ischaemic heart disease). Subjects who gave a history of intake of medicines, were known diabetics, were not observing proper medical fasting, were inpatients, were pregnant or had some physical or mental stress were excluded from the study.
Data collection
After explanation of the study prerequisites and obtaining a signed written consent, 172 subjects were sampled for fasting blood glucose. After the blood samples were taken subjects were requested to undergo a complete a 2-hour 75-g glucose challenge. They were asked to drink the glucose solution (300 mL) over 5 minutes. Starting from the time of intake of glucose, subjects were advised to return for the 2-hour sample for glucose. All samples were analysed within 3 (± 1) hours. Glucose was analysed by the hexokinase method on a random access clinical chemistry analyser (Hitachi-902).
Subjects were grouped into 5 groups according to their fasting blood glucose results: < 5.0 mmol/L ( 6.9 mmol/L (125 mg/dL).
Subjects were defined from 2-hour OGTT results as: normal glucose tolerance 199 mg/dL).
Statistical analysis
All data were entered into SPSS, version 15. Measures of central tendency and dispersion i.e., mean and standard deviation (SD) was calculated for age. Frequencies by sex were also calculated. Frequency of normal glucose tolerance, IGT and DM based upon the 2-hour OGTT readings were measured at various levels of fasting blood glucose defined cut-offs by utilizing descriptive statistics. The differences in 2-hour post-75-g glucose challenge based diagnosis, i.e. normal, IGT and DM across various levels (defined groups), were compared by 1-way ANOVA. Level of agreement between WHO and ADA criteria was measured by the kappa statistic.
Results
The mean age of our study population was 43.7 (SD 10.8) years. Out of the evaluated subjects, 139 were male and 33 were females.
Participants with IGT and DM based on the 2-hour OGTT results spanned all the ranges of fasting blood glucose results (Figure 1 ). An increasing frequency of diagnosis of IGT and DM was seen after the fasting blood glucose results crossed the level of 5.6 mmol/L.

Table 1 shows that the mean 2-hour OGTT results crossed the WHO and ADA defined cut-off of 7.8 mmol/L in subjects having fasting blood glucose results > 5.6 mmol/L.
The relative percentage of diagnosis rates of IGT and DM based upon 2-hour OGTT increased with age (Figure 2).

A total of 40.6% of subjects (29.7% IGT and 10.9% DM) with fasting blood glucose between 5.6–6.0 mmol/L had abnormal glucose regulation on the basis of the gold standard 2-hour OGTT results (Figure 3). The agreement between the ADA and the WHO diagnostic criteria was only fair (kappa = 0.32, P < 0.001).

Discussion
Our study has shown that subjects having fasting blood glucose < 6.1 mmol/L can have biochemically abnormal response in terms of glucose intolerance after subjecting them to a glucose load. A review of the literature revealed other studies that agree with our findings [11,13]. We also showed that even subjects in the normal range of fasting glycaemia, i.e. fasting blood glucose < 5.6 mmol/L, can have glucose dysregulation demonstrated during a 2-hour glucose challenge test. Therefore the current diagnostic approach may miss as many as 40% of cases with abnormal glucose metabolism as depicted by a 2-hour OGTT reading, i.e. IGT and DM. Similar results have been shown by studies in which subjects with normal glucose levels and abnormal 2-hour glucose load readings [14]. Richard et al. showed no reliable cut-off for fasting glucose was sensitive enough to rule out DM, and concluded that OGTT testing must be maintained for the diagnosis of DM [8].
The possible reasons for such findings could be multifold. First, fasting and post-glucose challenge results demonstrate 2 different processes; the former may reflect the glycaemic baseline being influenced by the amount of fasting and associated physical and psychological state, while the later reflects a stimulated response from the beta cells of pancreas after glucose loading [15]. Traditionally, stimulated or dynamic glucose testing has been referred as the gold standard. So both glucose results represent different dimensions to the diabetogenic processes [15–17]. Secondly, the trade-off between sensitivity and specificity of the test is the underlying consideration once decisions are required for screening or devising a confirmatory methodology [18]. Our results suggest that sole reliance on fasting blood glucose will compromise sensitivity due to the high number of false negative cases. Once a 2-hour OGTT reading is used, it not only adds strength to the diagnosis but also adds to the sensitivity by identifying subjects with glucose intolerance who have underlying atherosclerosis-related complications [19]. Thirdly, 2-hour readings have been shown to be better correlated with markers of glycation including glycosylated haemoglobin and fructosamine [20]. So why not incorporate a single 2-hour reading to screen subjects suspected of having DM? The quality and cost added by early diagnosis of DM can be weighed against the cost of late initiation of treatment. Already the literature recommends the use of OGTT in the detection of undiagnosed DM in certain disease categories such as stroke and myocardial infarction [21,22]. Philips et al. have recommended a glucose challenge test to improve the diagnostic sensitivity for undiagnosed DM [23]. The question arises as to why wait for the development of complications whose treatment cost greatly exceeds the screening cost? Finally, a review of certain regional literature on diabetes screening highlights the varying recommendations about screening in our population in contrast to their non-Asian counterparts [12,24]. More regional literature do exist recommending a lower threshold of screening for DM in our population [24–26]. Thus racial and regional differences in the pattern of diabetes could be another reason for subjects demonstrating glucose intolerance during glucose loading.
Comparing WHO criteria for diagnosis of diabetes with ADA criteria shows minimal agreement between the 2 definitions of similar disease. The available literature review does suggest comparable results [7,8,27]. In the opinion of the authors, the lower cut-offs may be more compatible with the screening concept for diagnosing DM, while subjects having a slightly higher degree of clinical suspicion may be further confirmed by adopting the 2-hour OGTT test as per WHO recommendations. There may also be an argument for further lowering the cut-offs for our population, but prospective clinical trials must address this issue [18].
We have also observed that ageing is related to post-glucose load intolerance. Choi et al. reported similar findings [12]. The reason could be the age-related deterioration in the functioning of beta cells of the pancreas or the development of insulin resistance which appears later in life [12,28].
There are some limitations to our study which should be noted. First, stress hyperglycaemia is a known entity. Attempts were made to exclude subjects with any degree of physical ailments, but a psychological stress evaluation was not carried out among our subjects. Secondly, the results may have been confounded by the presence of obesity, hypertension or ischaemic heart disease.
This study was clinically important because it highlighted controversies associated with the diagnostic criteria for DM. At present clinical practice suggests labelling of individuals as normoglycaemic based on a fasting blood glucose result. However, in the light of our findings it can be suggested that a patient can have DM when the fasting blood glucose result is normal. Interpretation of fasting blood glucose results must include consideration of a patient’s age and clinical information. Moreover, a 2-hour OGTT reading should also be considered as a screening test after a necessary cost–benefit analysis and consensus among the authorities.
Conclusion
Abnormalities of glucose metabolism including impaired glucose tolerance and DM can exist at fasting blood glucose results < 6.1 mmol/L (110 mg/dL). WHO and ADA criteria showed poor agreement between each other for the diagnosis of DM.
References
- Farag YM, Gaballa MR. Diabesity: an overview of a rising epidemic. Nephrology, Dialysis, Transplantation, 2011, 26:28–35.
- Chang K. Comorbidities, quality of life and patients' willingness to pay for a cure for type 2 diabetes in Taiwan. Public Health, 2010, 124:284–294.
- Baldé NM et al. Frequency of diabetic microangiopathy in newly diagnosed diabetes mellitus in Conakry: late diagnosis and lack of screening. Dakar Med., 2007, 52:165–170.
- National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes, 1979, 28:1039–1057.
- Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia. Report of a WHO/IDF consultation. Geneva, World Health Organization, 2006.
- Summary of revisions for the 2010 clinical practice recommendations. Diabetes Care, 2010, 33(Suppl. 1):S3.
- Herdzik E et al. Comparison of ADA and WHO diagnostic criteria for diabetes diagnosis and other categories of glucose intolerance. Polski Merkuriusz Lekarski, 2002, 13:316–320.
- Richard JL et al. Diagnosis of diabetes mellitus and intermediate glucose abnormalities in obese subjects based on ADA (1997) and WHO (1985) criteria. Diabetic Medicine, 2002, 19:292–299.
- Tirosh A et al. Normal fasting plasma glucose levels and type 2 diabetes in young men. New England Journal of Medicine, 2005, 353:1454–1462.
- Haffner SM et al. Cardiovascular risk factors in confirmed prediabetic individuals. Does the clock for coronary heart disease start ticking before the onset of clinical diabetes? Journal of the American Medical Association, 1990, 263:2893–2898.
- Balion CM et al. Reproducibility of impaired glucose tolerance (IGT) and impaired fasting glucose (IFG) classification: a systematic review. Clinical Chemistry and Laboratory Medicine, 2007, 45:1180–1185.
- Choi KM et al. Comparison of ADA and WHO criteria for the diagnosis of diabetes in elderly Koreans. Diabetic Medicine, 2002, 19:853–857.
- Kuzuya T et al. Committee of the Japan Diabetes Society on the diagnostic criteria of diabetes mellitus. Report of the Committee on the classification and diagnostic criteria of diabetes mellitus. Diabetes Research and Clinical Practice, 2002, 55:65–85.
- De Vegt F et al. Relation of impaired fasting and post load glucose with incident type 2 diabetes in a Dutch population: The Hoorn Study. Journal of the American Medical Association, 2001, 285:2109–2113.
- Li HY et al. The performance of risk scores and hemoglobin A1c to find undiagnosed diabetes with isolated post load hyperglycemia. Endocrine Journal, 2011, 58:441–448.
- Temelkova-Kurktschiev TS, Hanefeld M. Oral glucose tolerance test: to be or not to be performed? Clinical Laboratory, 2002, 48:143–152.
- Silverman RA et al. Hemoglobin A1c as a screen for previously undiagnosed prediabetes and diabetes in an acute-care setting. Diabetes Care, 2011, 34(9):1908–1912.
- Waugh N et al. Screening for type 2 diabetes: literature review and economic modelling. Health Technology Assessment, 2007, 11(17):iii–iv, ix–xi, 1–125.
- Hanefeld M et al. Post-challenge hyperglycaemia relates more strongly than fasting hyperglycaemia with carotid intima-media thickness: the RIAD Study. Risk Factors in Impaired Glucose Tolerance for Atherosclerosis and Diabetes. Diabetic Medicine, 2000, 17:835–840.
- Rosediani M, Azidah AK, Mafauzy M. Correlation between fasting plasma glucose, post prandial glucose and glycated haemoglobin and fructosamine. Medical Journal of Malaysia, 2006, 61:67–71.
- Lindsberg PJ, Tuomi T, Kaste M. Oral glucose tolerance test should be performed after stroke and transient ischemic attack. International Journal of Stroke, 2011, 6:317–320.
- Kitada S et al. Post-load hyperglycemia as an important predictor of long-term adverse cardiac events after acute myocardial infarction: a scientific study. Cardiovascular Diabetology, 2010, 9:75.
- Phillips LS et al. Glucose challenge test screening for prediabetes and undiagnosed diabetes. Diabetologia, 2009, 52:1798–1807.
- Noda M et al. Fasting plasma glucose and 5-year incidence of diabetes in the JPHC diabetes study—suggestion for the threshold for impaired fasting glucose among Japanese. Endocrine Journal, 2010, 57:629–637.
- Ryu S et al. Should the lower limit of impaired fasting glucose be reduced from 110 mg/dL in Korea? Metabolism: Clinical and Experimental, 2006, 55:489–493.
- Nang EE et al. Is there a clear threshold for fasting plasma glucose that differentiates between those with and without neuropathy and chronic kidney disease? The Singapore Prospective Study Program. American Journal of Epidemiology, 2009, 169:1454–1462.
- Park KS et al. Comparison of glucose tolerance categories in the Korean population according to World Health Organization and American Diabetes Association diagnostic criteria. Korean Journal of Internal Medicine, 2000, 15:37–41.
- Hasegawa G. Decreased senescence marker protein-30 could be a factor that contributes to the worsening of glucose tolerance in normal aging. Islets, 2010, 2:258–260.












